UNIVERSAL NASAL VACCINE PLATFORM FOR INFECTIOUS DISEASES
FINVAC Vaccine Technology
A new generation of intranasal vaccines
The first vaccine developed using this platform, FINVAC COVID-19, is a nasal vaccine against SARS-CoV-2 and is preparing to enter its second clinical trial.
Why intranasal vaccination matters
Key features of the FINVAC platform
- Rapid adaptability: A novel adenoviral vector backbone enables quick updates to address emerging variants and future infectious threats.
- Scalable production: Manufactured using a proprietary suspension cell line optimized for RCA-free, high-yield, and rapid adenoviral vector production.
- Enhanced formulation: A highly immunogenic vaccine buffer currently in clinical development.
- Easy administration: Simple nasal delivery promotes high compliance and supports self-administration, thus ideal for mass vaccination and low-resource settings.


Pipeline
Clinical Trials
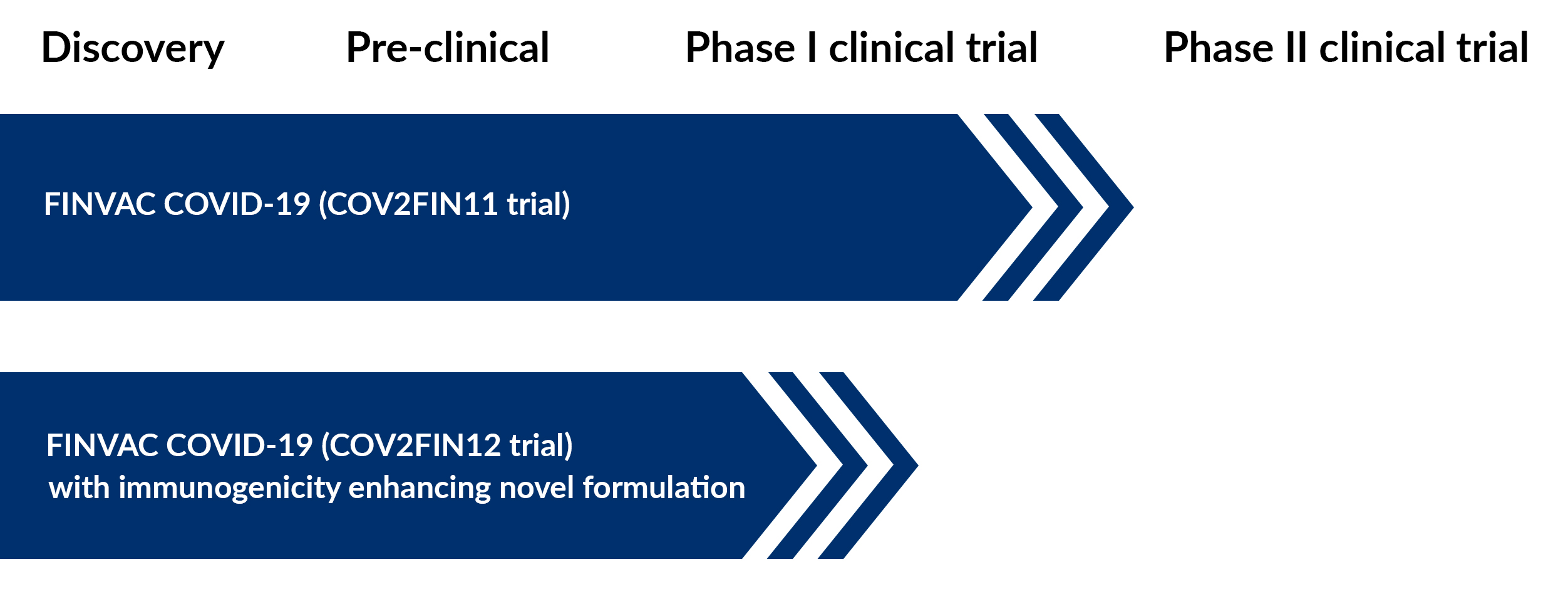
COV2FIN11 Phase I Trial
Rokote Laboratories has successfully completed its first Phase I clinical trial, assessing the safety and immune response of FINVAC COVID-19 delivered via two methods: nasal drops and nasal spray. Further analysis of the vaccine’s immunogenicity is currently underway.
COV2FIN12 Phase I clinical trial
The COV2FIN12 Phase I clinical trial using FINVAC COVID-19 is scheduled to start in January 2026, introducing an enhanced FINVAC platform with a novel buffer optimized for nasal delivery. This next-generation formulation is designed to position FINVAC as the leading nasal vaccine platform for pandemic preparedness and protection against seasonal respiratory pathogens.


About Rokote Laboratories Finland Oy
Our mission is to make vaccination safer, easier, and more equitable worldwide by developing affordable, needle-free, intranasal vaccines that block infection and transmission at the source.
Latest news
Rokote Laboratories Finland receives a second clinical trial authorization for FINVAC COVID-19 vaccine
Rokote Laboratories Finland Oy is proud to announce that we have received Clinical Trial Authorization (CTA) for our second Phase I clinical trial of the intranasal COVID-19 vaccine. The upcoming study, titled:"A Phase 1a, single-blind, randomized comparative clinical...
Terhi Reunama Appointed as Head of Clinical Trial Operations
We are pleased to announce the appointment of Terhi Reunama as our new Head of Clinical Trial Operations. Terhi brings nearly 30 years of clinical research experience from both the pharmaceutical industry and CRO organizations. A registered nurse with a Bachelor of...
Our Nasal COVID-19 Vaccine Moves to First Human Trials
Rokote Laboratories Finland Oy is starting the first clinical drug trial with its nasal COVID-19 vaccine. The vaccine has been developed and manufactured in Finland, and the study will be conducted at Kuopio University Hospital starting January 21, 2025. The...
Our Team
Key Personnel

Erkko Ylösmäki
CEO
BIO

Terhi Reunama
Head of Clinical
Trial Operations
BIO

Tobias Freitag
Head of Vaccine Immunity
BIO

Tuula Wallén
Quality Manager
BIO

Kirsi Hellström
CMC Advisor
BIO

Nora Rauhala
BIO

Sammeli Liikkanen
BIO
Board and advisors

Seppo Ylä-Herttuala
Chairman of the Board
BIO

Kalle Saksela
MD, PhD, Professor
Member of the Board
BIO

Aki Prihti
Member of the Board
BIO

Tuija Keinonen
BIO

Kari Alitalo
Scientific Advisor
BIO

Pasi Kemppainen
Strategy Advisor
BIO

Kari Varkila
MD, PhD, MBA
Clinical Development Advisor
BIO

Jenni Vuola
Business Development Advisor
BIO

Laura Suoranta
CMC Advisor
BIO

Anna-Kaisa Lehtivarjo
Quality Advisor